| Silviamar: Vegetables, chemistry and colour |
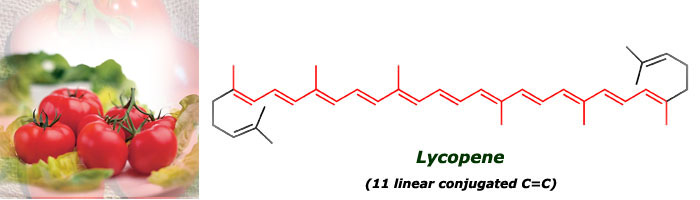
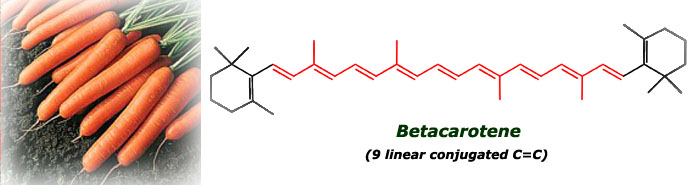
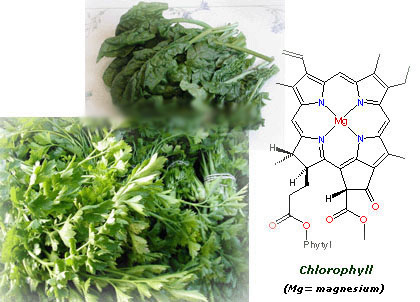
Category: Science for everybody 15 comments 16 Oct 2005 @ 18:00 by melztripp : i've just added a brain fold!!!hey sylvia, i absolutely love to learn things like this...which is why i should have been a chemist instead of a nurse. i think we spoke about this before. unfortunately, there is only 1 or 2 people that i can explain this to, who will actually care!!! and the ones i tell, will probably already know this anyway!! oh well, thanks for the chemistry lesson. hope all is well with you, melanie! ************* Hi Melanie! Yes, we've talked about it, and I'm pretty sure that you're learning many things on your own in this field. I always wanted to know about this things, I still remember getting shocked one day when I was 8 because I read the ingredients of a coconut cookies and realized that they didn't have any coconut but they tasted to it! :-) 16 Oct 2005 @ 18:33 by jstarrs : I just wish I'd had Silvia as a chemistry teacher when I was younger. The only kicks I got back then in chemistry were making stink bombs... I don't know the color of stink bombs. ********** Thank you Jeff. Well, you were more fortunate than me, I didn't learn to make stink bombs at school. Probably the teacher knew that if we learnt to make them, we would have used them in the class all the time :-D 29 Nov 2005 @ 15:05 by JMG @156.35.192.3 : teaching Silvia, are you a teacher? I wish all teachers explained as clearly as you, you could do a living teaching chemistry... ********* Thank you, you're very kind :-). Actually, I teach Chemistry at university, though I mainly do research. Teaching is what motivates me more. 20 Dec 2005 @ 15:41 by Ruthie @80.230.216.7 : lycopene uv- visible spectrum Hello I'm trying, in vain, to find the uv-visible absorption spectrum of lycopene. I would appreciate receiving a relevant link or the spectrom itself if you have it thanks Ruthie 21 Dec 2005 @ 00:38 by silviamar : Ruthie, this is the light absorption spectrum of lycopene at room temperature: {link:http://www.biophysj.org/cgi/content/full/87/5/3010/FIG11|Link} Hope it helps! :-) 23 Apr 2006 @ 08:12 by bachhue @125.234.65.217 : Lycopene Would you give me a UV vis spectrum of Lycopene Thank you very much ******** Hello Bachue, in the message just above yours I gave the link to the spectrum of Lycopene between 340- 560nm ({link:http://www.biophysj.org/cgi/content/full/87/5/3010/FIG11|Link}), with the main absorbance peaks at 446, 476 (the max absorbance) and 504 nm. I don't have it below that range. By the way, there is a great free NMR, IR and UV spectra database of many compounds here: {link:http://www.aist.go.jp/RIODB/SDBS/cgi-bin/cre_index.cgi|Link}, lycopene is not there, but it can be useful for you for other compounds. 18 Jun 2006 @ 12:11 by Joost @145.53.55.157 : lycopene NMR spectrum I'm looking for a nmr spectrum of lycopene Do you know where to find it? thank you ****** You can find the 1H-NMR here: {link:http://phot.allenpress.com/photonline/?request=get-document&doi=10.1562%2F0031-8655(2001)074%3C0444:TDCITC%3E2.0.CO%3B2} It's in table 2, first column. And one of the references included there (Helv. Chim. Acta. 75, 1848–1865) gives you the characterization of all the isomers of lycopene. 1 Nov 2007 @ 09:57 by adinarayana mundra @202.63.102.42 : chemistry I do make an effort to understand lycopene antioxidant activity 9 Jan 2008 @ 01:15 by Sydney @12.216.169.160 : Thanks!! this really came in handy with one of my chemistry project, you are officially my hero 29 Jan 2015 @ 06:51 by Will @186.88.224.4 : jMfWVWodXCYfbfjB Hi EdwinThanks for your question. The main reosan I don't believe in supplements is that our bodies are designed to extract the nutrients we need from the food we eat. That is the task our digestive system is designed to do. If you provide enough vitamins and minerals to the body in the food you eat, then supplements should not be necessary. If you take supplements in this case, then the body will flush the superflous vitamins and minerals out. Furthermore, scientific research continues to disagree on how effective supplements are. It appears that a few for example, B12, Folic Acid, Omega 3 oil are effective, but, again, if you are eating right, then you don't need them. Overall, it's generally better in my view to eat the right food than to take a pill.Thanks, Greg 29 Jan 2015 @ 18:23 by Jessica @213.183.76.226 : AOtdwyicivsebWP I can definitely get nthguay all up on that lasagna! My favorite Italian foods are meatballs and pesto. Meatballs were a Sunday staple growing up in my big Sicilian family. Oddly, pesto was never part of the mix and I discovered it later in life. Now I am married to a sweetheart of a Turk and make a dish that respresents both of our ethnicities: lamb meatballs with pesto yogurt. 30 Jan 2015 @ 06:50 by Natali @190.38.83.223 : puHcBdhQEjnTDW I think you've just captured the answer peetfcrly 5 Mar 2015 @ 09:25 by qrgl-technology @182.186.141.77 : I really enjoy simply reading all of you I really enjoy simply reading all of your weblogs. Simply wanted to inform you that you have people like me who appreciate your work. Definitely a great post 9 Mar 2015 @ 06:06 by v-travelled @182.186.221.151 : This is a smart blog. I mean it. You hav This is a smart blog. I mean it. You have so much knowledge about this issue, and so much passion 30 Mar 2015 @ 06:05 by c3caregiver @39.36.33.187 : I like you recommendation. Your recommen I like you recommendation. Your recommendation is of well use to people. A great article post, this is something very interesting. I really appreciate your post. Other entries in Science for everybody 4 Feb 2006 @ 14:42: Lactose intolerance 20 Nov 2005 @ 11:29: Why do onions make you cry? 17 Jul 2005 @ 09:27: How does soap clean?
|
 16 Oct 2005 @ 17:02, by Silvia M.S
16 Oct 2005 @ 17:02, by Silvia M.S